The formal charge of nitrogen in the compound NOis plus 1. The whole nitrate ion carries a total charge of minus when combining the charges of the one . Nitrate is a polyatomic ion with the molecular formula NO− and a molecular mass of 62.
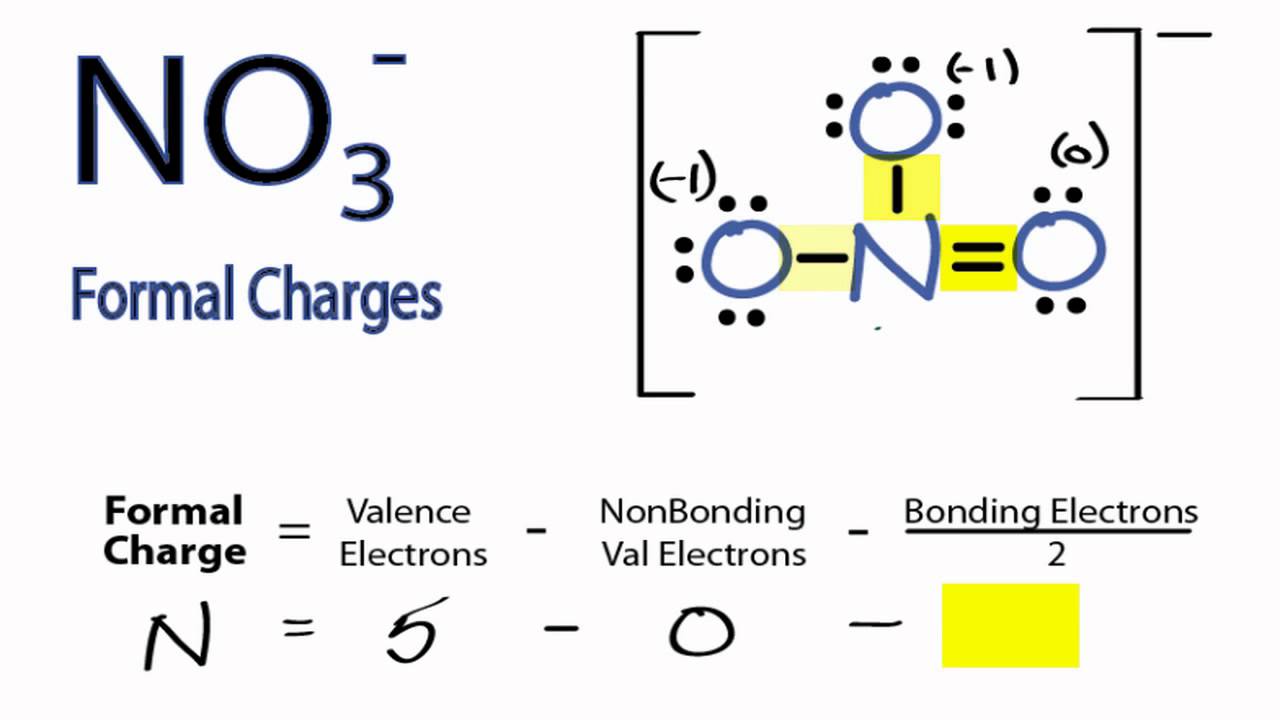
This from a combination formal charge in which each of the three oxygens carries a − 2⁄charge, whereas the nitrogen. NO3- (inorganic nitrate) is the viable active component within beetroot juice and other vegetables. The charge of each separate (NO3) ion is -1.
The charge of both total (NO3)ions is -2.
How do you find the charge of an ion like NO3? Jan 2012What is the formal charge for NO3- if you have a combined. Oct 2011Does NOhave the same charge as NO3?
Jan 2010Why is the total charge of Al(NO3)six? These ions are separated by charge on the ion into four (4) different tables and listed alphabetically within each. The simple answer to that is that the ionic form is more stable than the neutral NO3 .
No comments:
Post a Comment
Note: only a member of this blog may post a comment.